Predictive Multimodal Biomarker Platform for Smarter, Faster CNS Drug Development
Harnessing AI and neuroimaging to segregate responders and non-responders before the trial begins — reducing development costs and time while improving success rates.
Our platform uses predictive biomarkers and AI to identify likely responders—and exclude non-responders and placebo responders—before the trial begins. This leads to smaller, faster, and lower-cost trials with a significantly higher probability of success.
Solution Overview
Our AI-powered solution provides a cutting-edge suite of biomarkers for CNS conditions in an integrated platform that combines multimodal data across the following key domains:
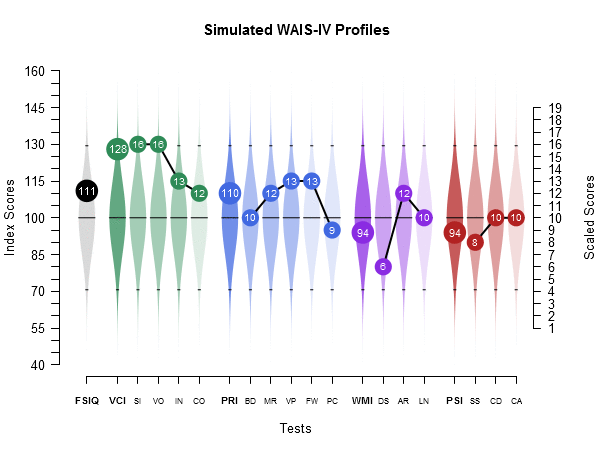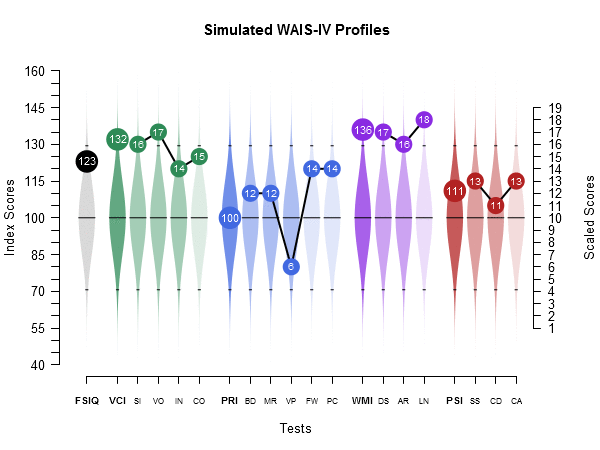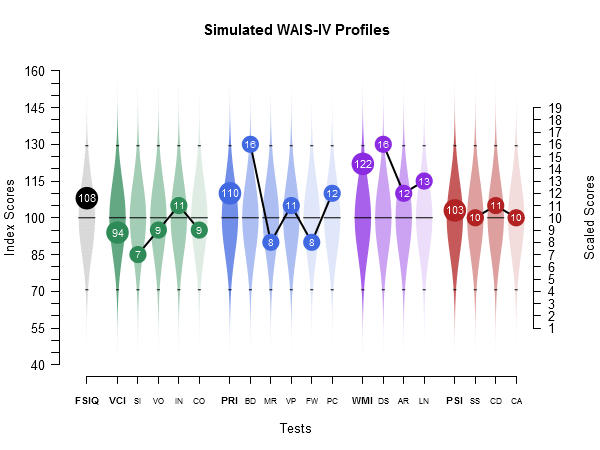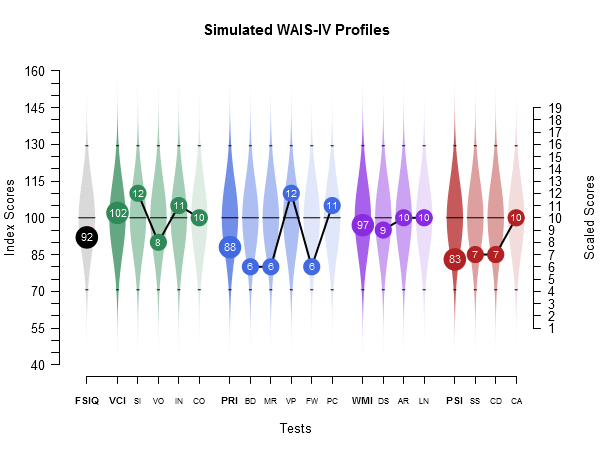
Cognitive Profile
Quantitative assessment of individual cognitive function, capturing attention, memory, executive function, and processing speed.
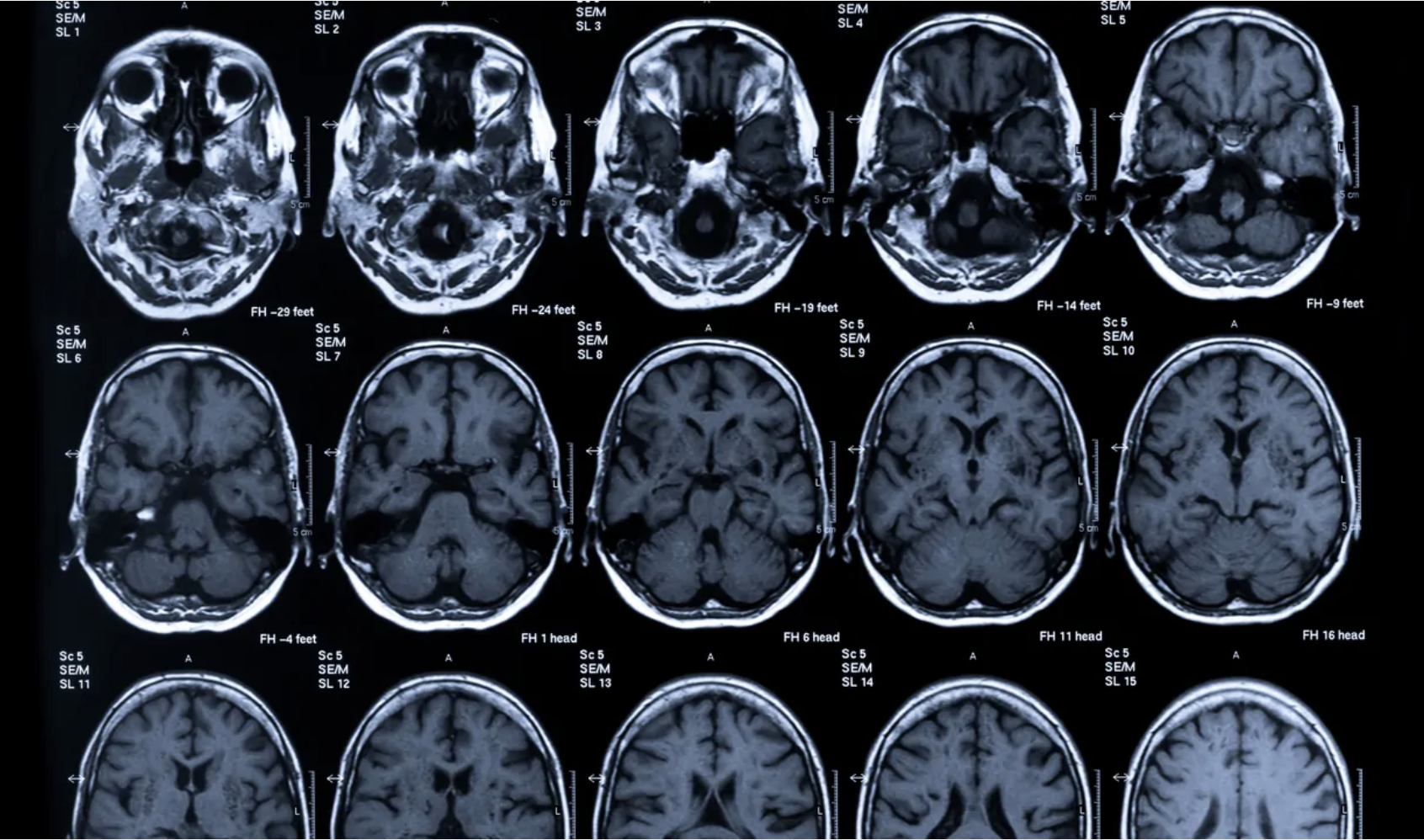
Brain Activity
Objective neural measures, including neuroimaging and brain markers, to provide insight into functional brain states.
Psychopathological, Clinical, and Demographic Measures
In-depth profiling of symptomatology, clinical history, and patient-specific characteristics to contextualize biological findings.
Clinically Impacted Biomarkers
Our markers are rigorously designed and tested, offering a holistic and scalable approach to stratifying patients into responders and non-responders thereby informing more targeted therapeutic development and thereafter, better clinical decisions. This platform bridges the gap between clinical observation and objective neurobiological measures, ushering in a new era of more precise drug development, diagnosis, treatment, and monitoring in CNS disorders.
Use Cases
Enrichment strategies for Phase II/III CNS trials
Precision recruitment to reduce trial duration
Rescue failing programs by identifying hidden responders
Why Are CNS
Trials Failing?
Lack of Patient Stratification
CNS disorders like schizophrenia, depression, and bipolar disorder are biologically heterogeneous and symptomatically overlapping. Traditional diagnostic categories often overlook neurobiological subtypes, leading to poorly targeted inclusion criteria and diluted treatment signals. Insel et al., 2010 describe this challenge in redefining psychiatric diagnoses through neuroscience.
High Placebo Response Rates
CNS trials—especially in depression and anxiety—are notoriously prone to high placebo response rates with meta-analyses showing placebo response rates exceeding 40% in modern trials (Rief et al., 2009; Papakostas et al., 2020).
This severely compromises the ability to detect treatment effects, increases the likelihood of trial failure even for biologically active compounds, and inflates development timelines and costs.
Subjective Endpoints and Trial Noise
Psychiatric assessments rely heavily on subjective clinician-rated scales (e.g., HAM-D, PANSS) or patient-reported outcomes, introducing variability and bias. Neuroimaging-based biomarkers offer objective, quantifiable endpoints that can reduce this noise and improve signal detection.
A smarter approach is needed.
Our platform directly addresses these pain points and is designed to reduce trial failure risk by offering neuroimaging-based biomarkers that stratify patients and predict individual placebo or treatment response. Our biomarkers are grounded in robust neuroscience, clinically interpretable, and regulator-ready.
Key Features
Cross-disorder applicability
Prospective model validation
Integration with EDC and trial workflows
Scalable across multi-site studies
We help sponsors enable more
decisive outcomes
Enrich for likely treatment responders
Exclude high placebo responders
Design smarter, smaller, more efficient trials
Get more decisive results
The result?
Smaller sample sizes, shorter trials, lower costs, and higher probability of success for innovative CNS therapies.
We empower drug developers with predictive tools that improve CNS trial success rates, helping bring effective therapies to market faster and aim to equip clinicians with neuroscience-powered tools to match patients to the most effective therapies—transforming trial-and-error treatment into personalized care.
The CNS Drug
Development
Crisis
Central nervous system (CNS) drug development has long been one of the most challenging areas in the pharmaceutical industry. Despite growing unmet medical needs in psychiatric and neurologic conditions, CNS drugs have the lowest probability of approval across all therapeutic areas.
Each failed trial can destroy $100M–$1B+ in value. This really slows down the ability to bring better therapies to patients in a timely fashion. And the burden of CNS conditions has grown tremendously:
>24M
patients worldwide
50%
partial improvement or unacceptable side effects
~$343B
economic burden in US
>280M
patients WW
70%
partial improvement with 1L SoC treatment
~$6T
economic burden to global economy by 2030
>55M
dementia patients WW, with up to 70% due to Alzheimer’s
50%
moderate stage by diagnosis; too late for disease-modifying treatment
12X
the economic burden of cancer in U.S. alone
About Us.
Redefining Success in CNS Clinical Trials and treatment
We envision a future where CNS disorders are treated with precision—where science personalizes care, clinical trials succeed more often, and patients get the therapies they need without trial and error.
Our Vision.
Our Mission.
"We combine predictive biomarkers, AI, and neuroimaging to bring precision medicine to neuroscience drug development."
We empower drug developers with predictive tools that improve CNS trial success rates, helping bring effective therapies to market faster and aim to equip clinicians with neuroscience-powered tools to match patients to the most effective therapies—transforming trial-and-error treatment into personalized care.
Our Team.
With over 45 years of combined expertise in psychiatry, neuroscience imaging, and therapeutic development, our team has been at the forefront of innovation in neuropsychiatry. Our legacy includes one of the earliest imaging studies to successfully predict patient response to a first-generation antipsychotic — over 30 years ago.
Since then, our team has designed and executed successful Phase I–IV clinical trials across multiple neuropsychiatric indications, such as pain, Autism, Schizophrenia, Bipolar disease, anxiety, including breakthrough programs in bipolar depression — a condition for which no FDA- or EMA-approved therapy previously existed and the only approved therapy for negative symptoms of Schizophrenia.
Our team also builds cutting-edge, AI-powered software that helps biopharma partners accelerate clinical development by understanding in-market and developmental stage products and optimizing endpoint selection, comparator arms, and statistical modeling — helping ensure that transformative therapies reach the patients who need them faster, while lowering the cost, increasing the likelihood of FDA approval and positioning the drug for commercial success.

Co-founder and Co-CEO
• Co-founded 4 companies, grew or managed annual revenues up to $200M and had a
successful exit.
• Experience building and growing talented teams from 0 to over 500 people.
• Partner KPMG, President & CEO, Xansa North America, Senior VP, Qwest
Communications.
• 35 years designing, building and executing technology, data and analytics driven
workflow and decision support solutions across various industries, including Life
Sciences, Consumer and industrial Products, Financial Services, Defense & Aerospace.

Co-founder and Co-CEO
• 20 years of diverse experience in CNS, Oncology, Ophthalmology, and Rare disease.
• Developed go-to-market strategy for a wide variety of pharmaceutical products such as
Pfizer’s Crizotinib, Genentech’s Tecenriq and Amgen’s Aimovig.
• Helped develop PharmaAcuity sales strategy and secured customers by identifying early
adopters & tailored messaging to meet specific pain points within their segments and
therapeutic conditions.
• At the Financial Times, performed investigative research to predict the success and
failure of key trials such as the CO 8 study of Avastin and United Therapeutic
Treprostinil.
News and Media.
Read about our media appearances, participation in industry events, knowledge articles and other news.
Coming Soon!
Publications on Precision Neuroscience

Developing an Electroencephalography-Based Model for Predicting Response to Antidepressant Medication

In Search of Biomarkers in Psychiatry: EEG-Based Measures of Brain Function

Selection criteria for neurophysiologic biomarkers to accelerate the pace of CNS therapeutic development
Scientific Publications
Authored by our team.
Coming Soon!
Let's Accelerate Your CNS Pipeline.
Our proprietary platform leverages machine learning to detect predictive signatures of treatment and placebo response in CNS disorders. We integrate structural and functional imaging with rigorous clinical data to deliver:
Treatment Response Predictors
Identify which patients are most likely to benefit from your investigational therapy boosting effect sizes.
Placebo Response Suppression
Minimize placebo responders, increase trial power, and reduce costs.
Stratified Trial Design Support
Enrich populations as per FDA guidance to improve study outcomes.
Interested in collaborating or
learning more about our platform?
Reach out to explore how we can help you enrich your trials, reduce costs, and get effective therapies to patients faster.
PA1.ai
Copyright © 2025 PA1.ai Inc.